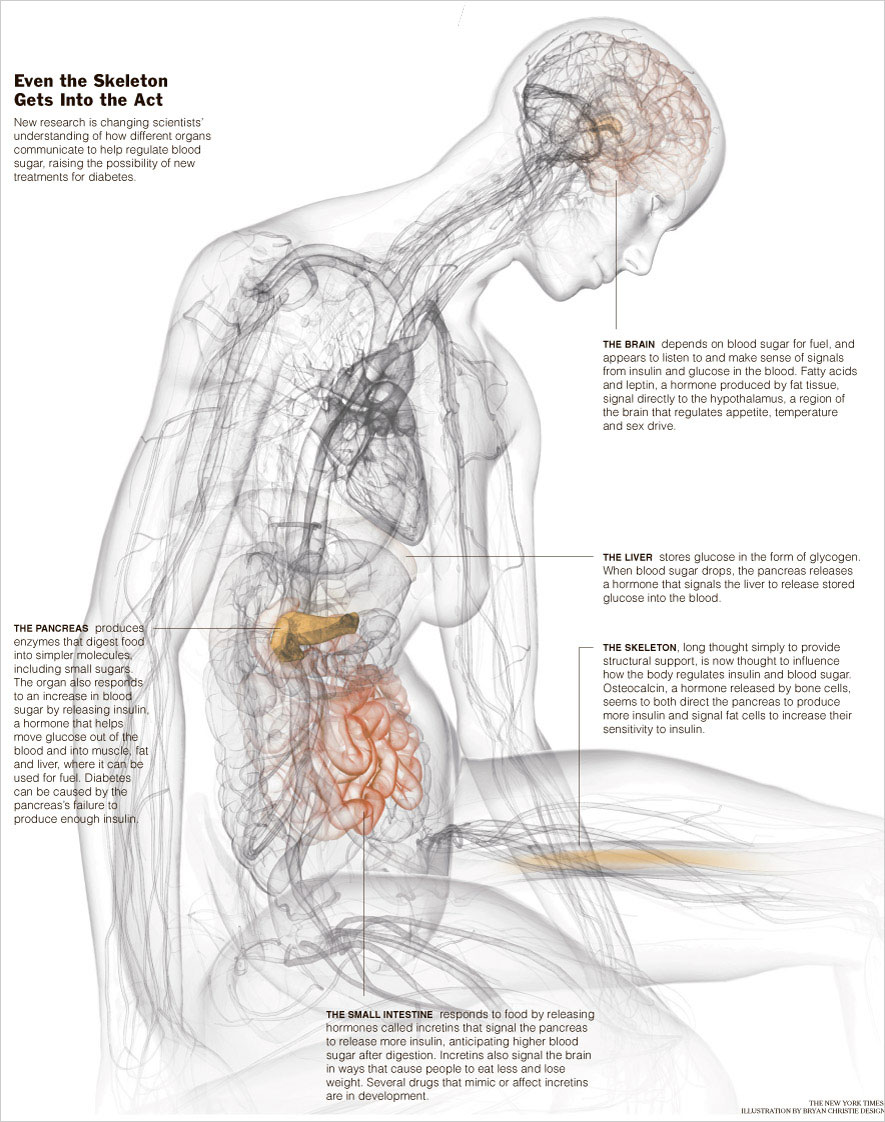
 |
||
|
| Want to send this page or a link to a friend? Click on mail at the top of this window. |
| More Science & Technogy |
| Posted October 17, 2007 |
 |
||
|
| In Diabetes, a Complex of Causes |
By AMANDA SCHAFFER |
An explosion of new research is vastly changing scientists’ understanding of diabetes and giving new clues about how to attack it.
The fifth leading killer of Americans, with 73,000 deaths a year, diabetes is a disease in which the body’s failure to regulate glucose, or blood sugar, can lead to serious and even fatal complications. Until very recently, the regulation of glucose — how much sugar is present in a person’s blood, how much is taken up by cells for fuel, and how much is released from energy stores — was regarded as a conversation between a few key players: the pancreas, the liver, muscle and fat.
Now, however, the party is proving to be much louder and more complex than anyone had shown before.
New research suggests that a hormone from the skeleton, of all places, may influence how the body handles sugar. Mounting evidence also demonstrates that signals from the immune system, the brain and the gut play critical roles in controlling glucose and lipid metabolism. (The findings are mainly relevant to Type 2 diabetes, the more common kind, which comes on in adulthood.)
Focusing on the cross-talk between more different organs, cells and molecules represents a “very important change in our paradigm” for understanding how the body handles glucose, said Dr. C. Ronald Kahn, a diabetes researcher and professor at Harvard Medical School.
The defining feature of diabetes is elevated blood sugar. But the reasons for abnormal sugar seem to “differ tremendously from person to person,” said Dr. Robert A. Rizza, a professor at the Mayo Clinic College of Medicine. Understanding exactly what signals are involved, he said, raises the hope of “providing the right care for each person each day, rather than giving everyone the same drug.”
Last summer, researchers at Columbia University Medical Center published startling results showing that a hormone released from bone may help regulate blood glucose.
When the lead researcher, Dr. Gerard Karsenty, first described the findings at a conference, the assembled scientists “were overwhelmed by the potential implications,” said Dr. Saul Malozowski, senior adviser for endocrine physiology research at the National Institute of Diabetes and Digestive and Kidney Diseases, who was not involved in the research. “It was coming from left field. People thought, ‘Oof, this is really new.’
“For the first time,” he went on, “we see that the skeleton is actually an endocrine organ,” producing hormones that act outside of bone.
In previous work, Dr. Karsenty had shown that leptin, a hormone produced by fat, is an important regulator of bone metabolism. In this work, he tested the idea that the conversation was a two-way street. “We hypothesized that if fat regulates bone, bone in essence must regulate fat,” he said.
Working with mice, he found that a previously known substance called osteocalcin, which is produced by bone, acted by signaling fat cells as well as the pancreas. The net effect is to improve how mice secrete and handle insulin, the hormone that helps the body move glucose from the bloodstream into cells of the muscle and liver, where it can be used for energy or stored for future use. Insulin is also important in regulating lipids.
In Type 2 diabetes, patients’ bodies no longer heed the hormone’s directives. Their cells are insulin-resistant, and blood glucose levels surge. Eventually, production of insulin in the pancreas declines as well.
Dr. Karsenty found that in mice prone to Type 2 diabetes, an increase in osteocalcin addressed the twin problems of insulin resistance and low insulin production. That is, it made the mice more sensitive to insulin and it increased their insulin production, thus bringing their blood sugar down. As a bonus, it also made obese mice less fat.
 |
PHOTOGRAPHS BY RICHARD PERRY/THE NEW YORK TIMES |
| DOWN TO THE BONES Working with mice, Dr. Gerard Karsenty of Columbia University found that a hormone related from bone may help regulate blood glucose. |
If osteocalcin works similarly in humans, it could turn out to be a “unique new treatment” for Type 2 diabetes, Dr. Malozowski said. (Most current diabetes drugs either raise insulin production or improve insulin sensitivity, but not both. Drugs that increase production tend to make insulin resistance worse.)
A deficiency in osteocalcin could also turn out to be a cause of Type 2 diabetes, Dr. Karsenty said. Another recent suspect in glucose regulation is the immune system. In 2003, researchers from two laboratories found that fat tissue from obese mice contained an abnormally large number of macrophages, immune cells that contribute to inflammation. The finding piqued the curiosity of researchers. “I remember reading the paper and thinking: ‘Wow, look at all those macrophages. What are they doing?’” said Dr. Jerrold M. Olefsky of the University of California, San Diego, School of Medicine.
 |
Scientists have long suspected that inflammation was somehow related to insulin resistance, which precedes nearly all cases of Type 2 diabetes. In the early 1900s, diabetics were sometimes given high doses of aspirin, which is an anti-inflammatory, Dr. Olefsky said.
Only in the past few years has research into the relationship of obesity, inflammation and insulin resistance become “really hot,” said Dr. Alan R. Saltiel, director of the Life Sciences Institute at the University of Michigan.
Many researchers agree that obesity is accompanied by a state of chronic, low-grade inflammation in which some immune cells are activated, and that that may be a primary cause of insulin resistance. They also agree that the main type of cell responsible for the inflammation is the macrophage, Dr. Saltiel said. Skip to next paragraph Health Guide Type 2 Diabetes Multimedia Graphic The Body’s Role in Diabetes
But major questions remain, he said: “Why are these macrophages attracted to fat, liver and muscle in the first place? What are they doing? What are they secreting? What other immune cells are in there?”
New research also suggests that “not all macrophages are created equal,” added Dr. Saltiel. There appear to be “good ones and bad ones” competing in fat tissue, with potentially large consequences for inflammation and diabetes.
Meanwhile, the promise of anti-inflammatory compounds as treatment continues to attract attention. “Certain cellular anti-inflammatory proteins may now be important new targets for drug discovery for diabetes treatment,” Dr. Olefsky said. But damping down the immune system is also potentially risky, he noted, adding: “If you’re inhibiting the macrophage inflammatory pathway, that’s good for insulin resistance and diabetes. But it might not be so good for your susceptibility to infections.” A major goal is to develop a drug that quashes only the specific component of macrophage inflammation that leads to insulin resistance, without causing other side effects.
One class of current medications, called thiazolidinediones, may work in part by reducing inflammation, which may in turn improve insulin sensitivity. But an example from this class, the drug Avandia, was also found to increase the risk of heart attacks.
Another participant in the glucose conversation is the brain. Its role has long been suspected. More than a century ago, the French physiologist Claude Bernard suggested that the brain was important in blood sugar regulation. He punctured the brains of experimental animals in specific areas and managed to derange their blood sugar metabolism, making them diabetic.
But for years, virtually no one followed up on this finding, said Dr. Kahn, of Harvard.
People thought about glucose as a critical fuel for the brain, Dr. Kahn said, but did not explore the brain’s role in glucose regulation.
Only recently, with more advanced laboratory techniques, has this role been definitively established and expanded upon.
Today’s genetic techniques, said Dr. Rizza, at the Mayo Clinic, are what have “really driven the process.”
For instance, once scientists developed the ability to manipulate mice so that they lacked particular receptors in specific tissues, they could show that mice without insulin receptors in the brain could not regulate glucose properly and went on to develop diabetes, said Dr. Kahn, whose laboratory published this groundbreaking work in 2000.
Other researchers have shown that free fatty acids, as well as the hormone leptin, produced by fat tissue, signal directly to a part of the brain called the hypothalamus, which also regulates appetite, temperature and sex drive.
And several recent papers suggest that direct signaling by glucose itself to neurons in the hypothalamus is also crucial to normal blood sugar regulation in mice.
“If the brain is getting the message that you have adequate amounts of these hormones and nutrients, it will constrain glucose production by the liver and keep blood glucose relatively low,” said Dr. Michael W. Schwartz, a professor at the University of Washington. But if the brain senses inadequate amounts, he continued, it will “activate responses that cause the liver to make more glucose, and new evidence suggests that this contributes to diabetes and impaired glucose metabolism.”
The brain, therefore, appears to be listening to — and weighing and making sense of — a chorus of signals from insulin, leptin, free fatty acids and glucose itself. In response, it appears to send signals to liver and muscle cells by way of several nerves, though additional mechanisms are probably involved. The gut also seems to chime in, said Dr. Rizza, adding that for him, this aspect of sugar regulation came as “the biggest gee whiz of all.”
“Food comes in through the gut, so of course you should look there” for molecules involved in glucose regulation, he said. “But few people realized this until very recently.”
Hormones from the small intestine called incretins turn out to talk directly with the brain and pancreas in ways that help reduce blood sugar and cause animals and people to eat less and lose weight, Dr. Rizza said.
Numerous molecules that mimic incretins or prevent them from being degraded are in clinical trials. Two such drugs have been approved by the Food and Drug Administration: Byetta, an incretin mimic, from Amylin Pharmaceuticals and Eli Lilly; and Januvia, from Merck, which inhibits the destruction of the incretin GLP1. (Dr. Rizza is an adviser to Merck but says all consulting fees go to the Mayo Clinic for education and research.)
Still, it can be hard to predict how different drugs will interact in the body. And many promising candidates will turn out to have side effects — chattering helpfully with one organ, but problematically with another.
“The picture is becoming more and more complicated,” Dr. Saltiel said. “And let’s face it, it was pretty complicated before.”
Copyright 2007 The New York Times Company. Reprinted from The New York Times, Science Times, of Tuesday, October 16, 2007.
| Wehaitians.com, the scholarly journal of democracy and human rights |
| More from wehaitians.com |